
How Voltage in Metals Is Created
<
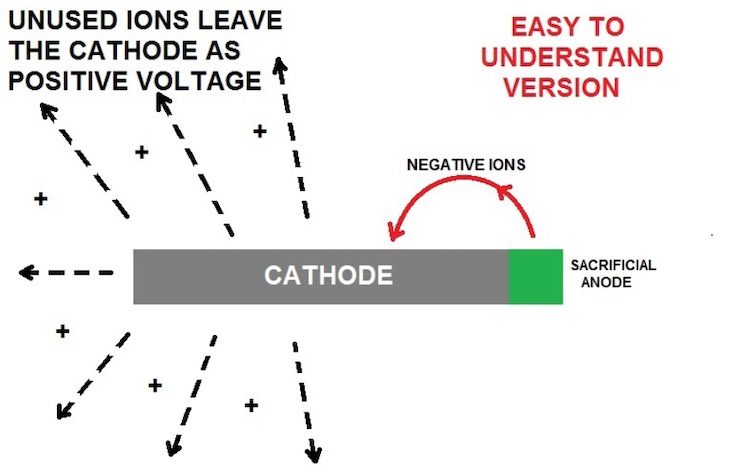
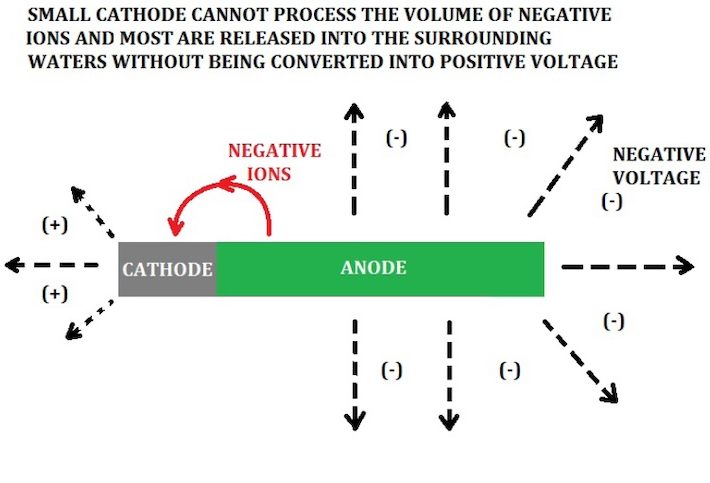
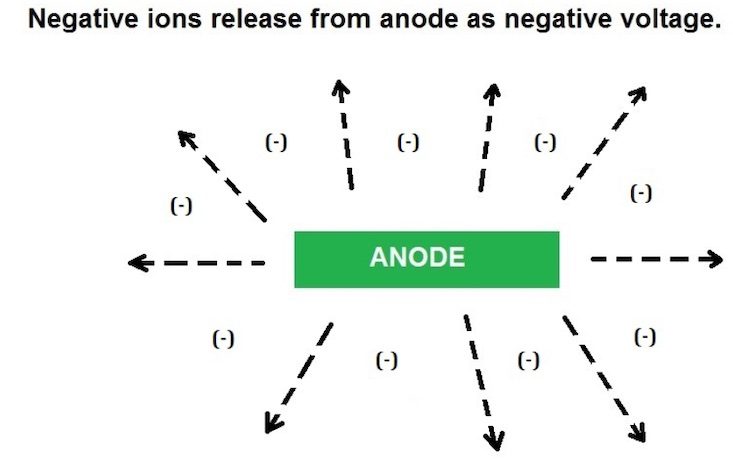
Virtually all metals are rated on the galvanic series with a negative voltage value, except for gold, which is zero. The more sacrificial the metal, the more negative voltage it will give up, also known as corrosion. When you connect these sacrificial anodes to a higher- rated metal (cathode), the ions from the anode bombard the cathode with the negative ions, and whatever is not needed to fully protect the cathode is released and dispersed into the water and converted into positive volts in the process. As an example, zinc is rated at -1.05 volts and marine stainless is rated at -.45 volts. That leaves you with a surplus of up to +.6 volts. This is subject mainly to the conductivity or mineral content of the water (electrolyte), as well as the proportions of the 2 metals. Most metals are alloys, so there can be many different ratings. Aluminum is a good example. You can have aluminum anodes on an aluminum hull and still have a hull reading in the +.5 volts range. My saltwater lure anodes are essentially a zinc alloy, while the spoons are nickel and stainless, and they will give off a positive value in the +.7 volts range in saltwater. Remember, if your anodes are not corroding, no positive voltage is being created. Future testing will include both aluminum and magnesium anodes.
Fish Attracted—The ‘Fishiest’ Boats Are Commercial Trollers & Unpainted Aluminum Hulls
 Fish Lightly Repelled—This Includes Most New Boats & Outboards
Fish Lightly Repelled—This Includes Most New Boats & Outboards
Fish Strongly Repelled
Zinc has a split personality. If it is by itself, or you have too much in relation to the metals to be protected, you end up with too many negative ions (volts) spilling and radiating into the water.
Don’t believe me? In 2012, I sent some of my lure tuning anodes to a friend in Northern Ontario to test on ice fishing for perch. He and five friends went out on Black Bay and drilled their holes in roughly a 50-ft circle. My friend and I had a communication problem and instead of attaching the 1⁄4” round zinc anode to his lure metals, he used it like a lead split shot weight and attached it to his fishing line about 2′ above his lure. For the first half of the day, he caught nothing. The two closest guys caught very little, while the three furthest away had decent catches. At about midday, he removed the anode completely and all catches became relatively equal. That “perch incident” was my first introduction to the fact that fish can be repelled by certain metals underwater. I have since made a Mini Inline Tuner that works awesome for ice fishing.
While doing my recent trout testing, and when my 14′ Lund was set up in attracting mode, I hung a piece of plain zinc off of the transom, attached with 10-gauge copper wire, so the zinc was electrically connected to and protecting the aluminum hull. This gave me about a 350-to-1 ratio of bare hull to zinc. The boat is essentially as neutral as possible with all engine zincs removed.
In order to switch the boat from attracting to repelling mode, the wire is replaced with twine, so there is no electric connection to the aluminum hull.
Fish Repelling Mode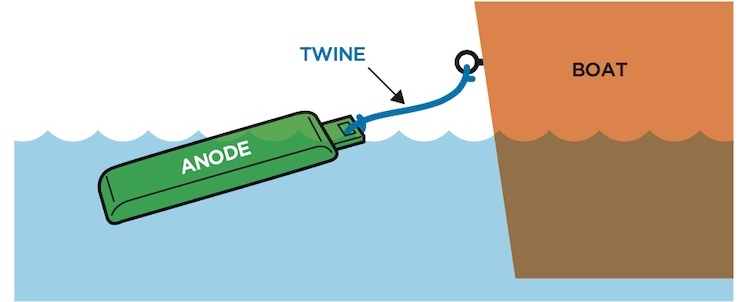
Fish Attracting Mode

I was contacted by four anglers in a row last summer. They had all replaced their old aluminum boats with new ones. They all complained that their fish catch rates went “in the dumps.” It turned out that their new boats had totally painted bottoms right from the factory. Guess what their old ones were? Bare, unpainted hulls! The math is easy. Their newer outboards all have excessive amounts of anodes, and there is almost no visible metal for the anodes to protect, resulting in repelling fish. About the same time, an owner of a fibreglass boat also contacted me. He had recently replaced his old Mercury outboard with a new Yamaha, loaded with anodes. His fishing went in the same direction as the aluminum boat guys. Once again, the zinc ratio is out of whack.
So how can you fix this? Change the ratio of anodes to hull/motor metals. Paint-sealed and fibreglass hulls or components will not help. You can remove some of the hull paint on aluminum boats, reduce a large portion of your anodes, or seal up a large section of them with paint or tape. I have tested extensively with my tuned lures and the lowest ratio where I still see good fishing results is 5 to 1 (1 being the anode). Even more cathode is better. The standard for ocean ships and steel barges is somewhere around 100 to 1. For most anglers, this will not be achievable, especially with saltwater boats, so try to achieve a ratio of 5 to 1 or better. You won’t be disappointed.
Common Anode Placement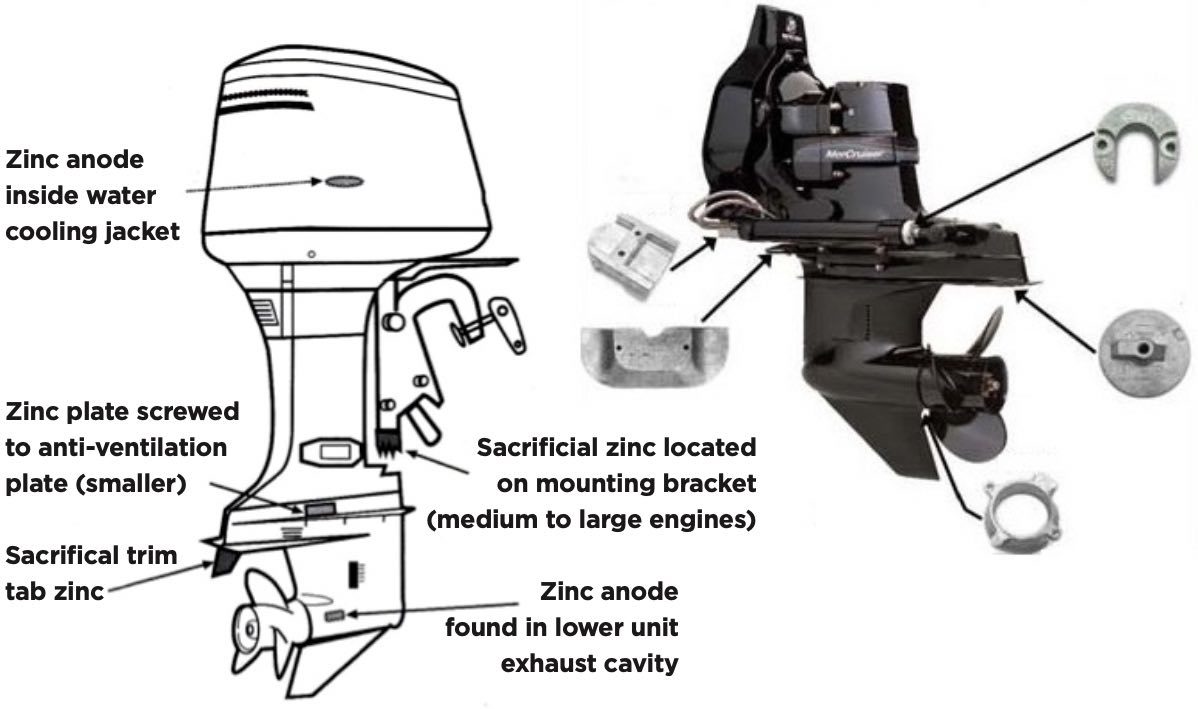
For oceangoing aluminum boats, a sealing epoxy is commonly used under the antifouling paint, resulting in a hull that will not interact with any anodes. Steel-hulled vessels are different, as the copper based antifouling paint actually absorbs water, allowing the steel to interact with the anodes.
Some of the other fixes include switching to a stainless prop or removing the paint from your aluminum one, but only if it is connected to the leg metals. You can also look at adding things like a stainless or aluminum cavitation plate or prop guard.
Bare Metal Cavitation Plate
Bare Metal Prop Guard
If equipped with stainless trim tabs, make sure that they are electrically connected (bonded) to your engine or leg anodes.
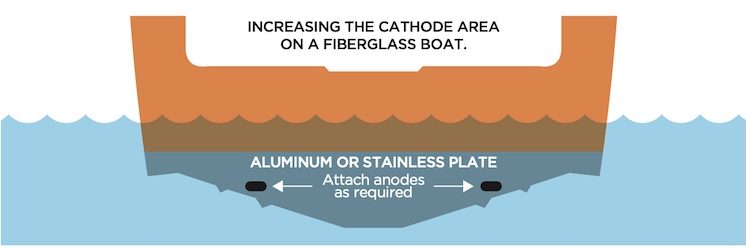
For fibreglass boats, an option is to add a stainless or aluminum plate to your hull or transom and connect it to your bonding system. Just don’t paint it!
Transom Plate
Allan Dampier is the founder of Lurecharge products, was a 4th generation commercial fisherman starting from the Great Lakes, and gravitating to the west coast in 1971. Over the years, he has owned and fished many boats, including 3 salmon trollers.
2 Comments
Leave A Comment
Visit the Store
$34.99
$34.99
Featured Catch

Joel Unickow halibut (Photo: Rob Frawley Lucky Strike Sportfishing Tofino)







I have afiberglass boat. Just checked the connections at a x10 , got about 300 Ohms between the kicker and the main engine, and about 60 Ohms between the immersed portion of the main motor and a ground on the main motor. Is that good? Any suggestions?
I am very interested to see how aluminum anodes would affect this equation. Have you done that testing? If so, have you published it anywhere?